In a groundbreaking fusion of biotechnology and nanotechnology, researchers have pioneered a revolutionary technique known as cellular-level embroidery, where microscopic robotic arms manipulate individual proteins with unprecedented precision. This emerging field, often referred to as protein stitching, leverages advanced micro-mechanical systems to assemble biological molecules into complex, functional structures. The implications for medicine, materials science, and synthetic biology are staggering, offering a glimpse into a future where human-engineered cellular architectures could outperform natural systems.
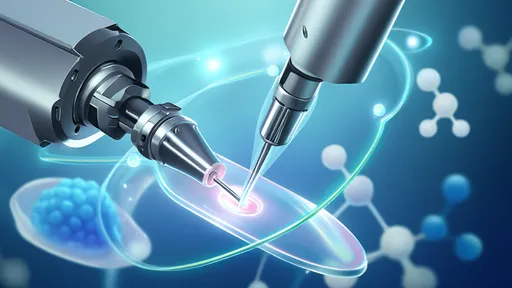
The core innovation lies in the development of nanoscale robotic manipulators, each smaller than a human cell, capable of handling proteins like microscopic needles threading molecular fabric. These devices operate in liquid environments, guided by real-time atomic force microscopy and machine learning algorithms that predict protein folding patterns. Unlike traditional genetic engineering, which relies on stochastic cellular processes, this approach allows for direct positional control at the angstrom level—essentially sewing together biomolecules with surgical accuracy.
One particularly striking application involves the creation of artificial extracellular matrices for regenerative medicine. By precisely arranging collagen, fibronectin, and growth factors in three-dimensional patterns that mimic native tissues, scientists have successfully guided stem cells to form functional organoids with vascular networks. Early experiments with diabetic wound models show 40% faster healing rates compared to conventional scaffolds, as the embroidered matrices provide both structural support and biochemical signaling cues in perfect spatial harmony.
The technology's origins trace back to cryo-electron microscopy breakthroughs in the late 2010s, which first enabled researchers to visualize protein structures with near-atomic resolution. This imaging revolution, coupled with advances in piezoelectric actuators and DNA origami techniques, laid the foundation for today's protein manipulation systems. Current platforms can position individual amino acid chains with 0.3-nanometer precision—a feat comparable to arranging grains of sand on a beach without touching neighboring particles.
Challenges remain in scaling up production, as the serial nature of protein stitching currently limits output to micrometer-scale constructs over several hours. However, recent developments in parallel nanorobotic arrays suggest this bottleneck may soon be overcome. A team at ETH Zurich recently demonstrated a 144-arm system working in concert to assemble a functional enzyme complex in under twenty minutes—a critical step toward industrial-scale biomolecular manufacturing.
Beyond medical applications, the technique shows promise for creating exotic biomaterials with properties unmatched in nature. Researchers at MIT have engineered light-emitting protein arrays that maintain quantum coherence at room temperature, while groups in Japan have developed bacterial-scale "molecular looms" that weave spider silk proteins into macroscopic fibers with ten times the toughness of Kevlar. These materials derive their extraordinary performance from the perfect alignment of every constituent molecule—an achievement impossible through self-assembly alone.
Ethical considerations accompany these technological leaps. The ability to rewrite cellular architectures at will raises questions about biocontainment and unintended ecological consequences. Leading laboratories have established stringent protocols, including protein-based kill switches and spatial confinement systems, but the broader scientific community continues to debate appropriate safeguards for this powerful new capability.
As the field matures, some visionaries speculate about programmable cellular ecosystems where nanorobotic assemblers maintain symbiotic relationships with living organisms. Theoretical designs include photosynthetic factories where chloroplast proteins are continuously repaired and optimized by microscopic maintenance crews, or immune systems augmented with molecular-scale sentries that detect and neutralize pathogens before infection occurs. While such scenarios remain speculative, they underscore the transformative potential of merging mechanical precision with biological complexity.
The coming decade will likely see protein stitching transition from laboratory curiosity to mainstream biotechnology tool. With major pharmaceutical companies already investing heavily in the technology for next-generation drug delivery systems, and defense agencies exploring its applications in adaptive protective materials, cellular-level embroidery appears poised to redefine what's possible at the intersection of life and machinery. As one researcher quipped, "We're not just playing God—we're playing Tailor."

By /Oct 22, 2025

By /Jul 25, 2025
By /Jul 25, 2025

By /Jul 25, 2025
By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025
By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025
By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025

By /Jul 25, 2025